The P cycle
When seeds are used for feed or food, their phytate is only available to ruminant animals. Therefore, phytate is excreted by monogastric animals (pigs, poultry, fish and humans), resulting in the P in their excreta causing environmental impacts such as eutrophication of water bodies. Seeds of sown crops contain less than 1 kg/ha P, so most crop P must be acquired from the soil. Soil P levels can be built by manures, or by fertilisers derived from rock phosphate. Soils commonly fix the majority of freshly applied P into forms that are unavailable to plants, so fertilised croplands accumulate soil P, adding to environmental impacts when soil sediments erode into water bodies.
Critical P levels in crops
P deficiency in crops causes a blue-green or purple tinge to leaves or stem bases and stunts growth. As P is mobile in plants, deficiency symptoms usually appear first in older leaves. Sufficient levels of P are essential for good establishment of crop seedlings; consequently, any deficiency will likely appear during emergence and early growth of annual crops or in spring in herbage.
Critical concentrations of P in healthy plant tissue are between 0.2-0.4% in dry matter and critical concentrations of P in wheat grain have been defined as 0.32%, based on multiple long-term field experiments dating back to the 1970s. Concentrations of P lower than this critical level indicate that yields are likely to have been reduced by inadequate P uptake. Fertiliser Recommendations now advocate use of Grain Nutrient Analysis to help monitor the P status of fields and crops.
Crop available P in the soil
Plant roots take up P only from soil solution, in the form of H2PO4- and HPO42-. However, the soil solution only holds a very small proportion of soil P reserves. The remaining P is locked up in readily available (small), less readily available (bigger), and very slowly available (biggest) soil P pools. Soil pH influences the availability of P in soil. Acidic soils cause phosphorus fixation by iron (pH 3-4) and aluminium (pH 5-6) whereas alkaline soils cause fixation of P by calcium (pH 8-9). The optimum pH for the highest P availability is close to pH 7.
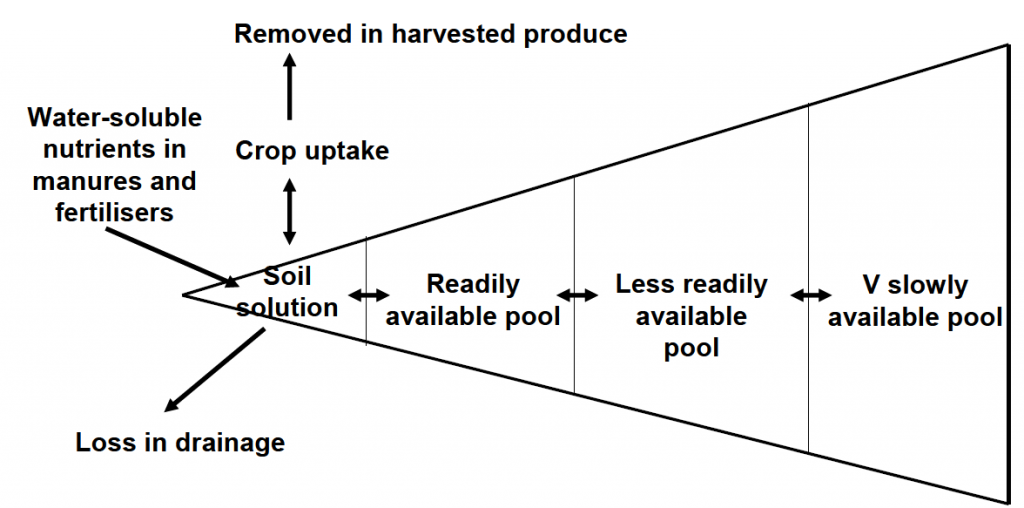
Despite crop available P in soils being the smallest pool of soil P reserves, only a minor part of any crop's P uptake comes directly from recently applied fertilisers. To ensure sufficient P availability from soil, regular monitoring of soil P is required. The Olsen method has been adopted for this in England & Wales, and a modified Morgan's method in Scotland. Advice is to maintain soil P at a target Index of 2 (16-25 mg/litre Olsen P) by replacing P that is removed in crop products (grains, straw, etc.) using either organic manures or synthetic fertilisers. Well-rooted soils, and soils with active micro-organisms (e.g., mycorrhizal) are critical to successful P nutrition of crops. Higher levels of organic matter improve soil structure and root exploration and can hold P in a readily available form.
Legacy soil P
Common manufactured P fertiliser sources are:
- Triple Superphosphate (TSP; 46% P2O5)
- Mono-ammonium phosphate (52% P2O5)
- Di-ammonium phosphate (46% P2O5)
Efficiency of plant uptake of applied P is subject of debate; immediate recovery from fresh fertiliser is less than 10%, but it is argued that 100% efficiency is represented by crop P offtake being exactly replaced by fertiliser P, and soil P levels not changing. However, recent research has shown that assumptions in fertiliser recommendations (RB209) about crop P contents have been exaggerated since the 1970s, hence (if available soil P has not increased), unavailable or "legacy" soil P must have increased, and 100% efficiency of fertiliser P has not been achieved. Defra-funded research has estimated annual P additions to UK soils to be ~300,000 tonnes per year, whereas annual removals are estimated at only ~200,000 tonnes.
Increases in 'legacy soil P' have large negative implications for environmental pollution as P is a key driver of eutrophication in our water courses, and the main cause of water quality failures. Also, despite soil additions, grain analysis of cereals has indicated that more than half of current crops are deficient in P, likely impacting yield. Consequently, better P management is urgently needed, specifically a shift to feeding the crop rather than feeding the soil. Improvements in P fertiliser availability and efficiency can be made through optimising the time of application; enhancing root architecture; good soil structure; placing P fertiliser close to the seed; and using forms of fertiliser that inhibit or by-pass soil P fixation, e.g., foliar fertilisation (see foliar nutrition topic).
For information on innovations, research and organisations involved in P nutrition its worth checking out the European Sustainable Phosphorus Platform.








Discussion
Soil analysis tells us that 80% of arable soils in E&W have adequate soil P (Index 2 or more).
But grain analysis over the last 6 years tells us that >50% of cereals are deficient in P (grain P <0.32%)
Should we be relaxed or worried about the adequacy of current cereal P nutrition?
Presumably it is a question of return on investment. 1) How efficient is any action taken to address the problem? 2) What is the return relative to cost?
Is there an opportunity to add a marker to phosphate to explore the uptake efficiency of different treatment sources and timings?
In the old days labs used P-32 to trace uptake of phosphorus, but modern H&S regulations (due of its radioactivity) now make this more problematic. However, see 1950s work on spring barley at Rothamsted here ... where they say "P recoveries at harvest ... varied ... from drilled (placed) superphosphate (10% — 15%) ... and from broadcast superphosphate (5% — 12%)". These recoveries are in-line with our recent estimates (averaging ~4% recovery from broadcast) using the difference method, because they imply that the fertiliser P reduced uptake of soil-derived P. However, I can't afford to buy the full article so I can't see their data and work out whether this is what they mean.
I want to find plants that will extract phosphate from low concentration sources. Any ideas?